26 March 2025
VOLTAIRE Clinical Trials - Plain Language Summary Now Available
A plain language summary is now available for the VOLTAIRE clinical trials. The trials compared adalimumab-adbm (a biologic drug) to the adalimumab reference product in people with rheumatoid arthritis, Crohn’s disease, and chronic plaque psoriasis.
The summary reports the overall long-term safety results. You download and read a PDF of the article published by Taylor & Francis for free, here. A brief overview of the summary can also be found below.
What is this summary about?
Adalimumab, also known by the brand name Humira®, is a biologic drug that is approved for the treatment of many autoimmune conditions including rheumatoid arthritis, Crohn’s disease, and chronic plaque psoriasis. Adalimumab-adbm, known by the brand name Cyltezo®, isa biosimilar to Humira®. While biologics are large, complex organic molecules made with living cells grown in culture, a biosimilar is a type of biologic that is highly similar to its reference product, or original biologic.
The VOLTAIRE clinical trials compared adalimumab-adbm to the adalimumab reference product in people with rheumatoid arthritis, Crohn’s disease, and chronic plaque psoriasis. This summary reports the overall long-term safety results of the 5 VOLTAIRE clinical trials that were part of the program (called VOLTAIRE-RA, VOLTAIRE-RAext, VOLTAIRE-CD,VOLTAIRE-PsO, and VOLTAIRE-X). This is known as a pooled analysis.
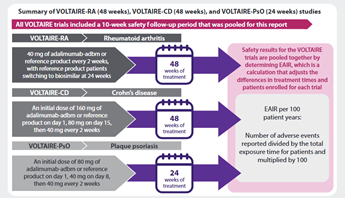 What were the results?
What were the results?

Overall, people who took part in the VOLTAIRE trials had very similar outcomes such as safety and drug effects when they switched from adalimumab reference product to adalimumab-adbm.
What do the results mean?
The results of this study show that in people with rheumatoid arthritis, Crohn’s disease, or chronic plaque psoriasis who took adalimumab-adbm and adalimumab reference product, a similar number of people experienced side effects or had to stop taking the treatment. These results show that people who took adalimumab-adbm should have no more side effects than if they were to take the adalimumab reference product, and vice versa.
What is adalimumab-adbm?
Adalimumab-adbm (Cyltezo®) is a biosimilar used to treat inflammatory autoimmune diseases such as rheumatoid arthritis, Crohn’s disease, and chronic plaque psoriasis. It is a biosimilar to the original biologic, also known as the reference product, adalimumab (Humira®)
What are biologics?
Biologic medications are specifically designed to mimic chemicals that are naturally found within the human body, and act to correct something that is going wrong. A well-known biologic treatment (that is not used for psoriasis) is Insulin, which is taken by diabetics. You can read more about them on our biologics webpage or by downloading our biologics for psoriasis and psoriatic arthritis information sheet.